FDA Validation Toolkit

FDA VALIDATION TOOLKIT SPEEDS THE PROCESS & REDUCES COSTS
GAMP 5 Guidelines describes in detail how cGMP requirements can be addressed by computerized systems and about the FDA validation strategy. As per GAMP 5, regulated companies have to document that GxP regulated systems (which keeps cGMP information) are compliant and fit well for the intended use. These guidelines define critical requirements expected of GxP computerized systems by building upon existing industry good practices in an efficient and effective manner.
FDA regulations say what a company should do, not how to do it. Without a set of templates to start with, many companies rely on their FDA validation service agency to write the necessary scripts, which adds time and cost to the validation process.
BatchMaster Software offers a set of validation templates in its FDA Validation Toolkit which satisfies FDA Guidance and ISPE GAMP® 5 best practices. These templates eliminate the need for the companies to write their own, thus accelerating the testing and validation processes, thus helping reduce validation services costs. The templates are kept in synch with FDA’s Validation best practices for documentation to reduce the risk of non conformance. For international businesses, the generated documentation is acceptable across multiple geographical boundaries, as they satisfy both FDA and ICH guidance’s (Q8, Q9 and Q10 ).
FOLLOW THE GAMP 5 SPECIFICATION, TESTING & VERIFICATION PROCESS
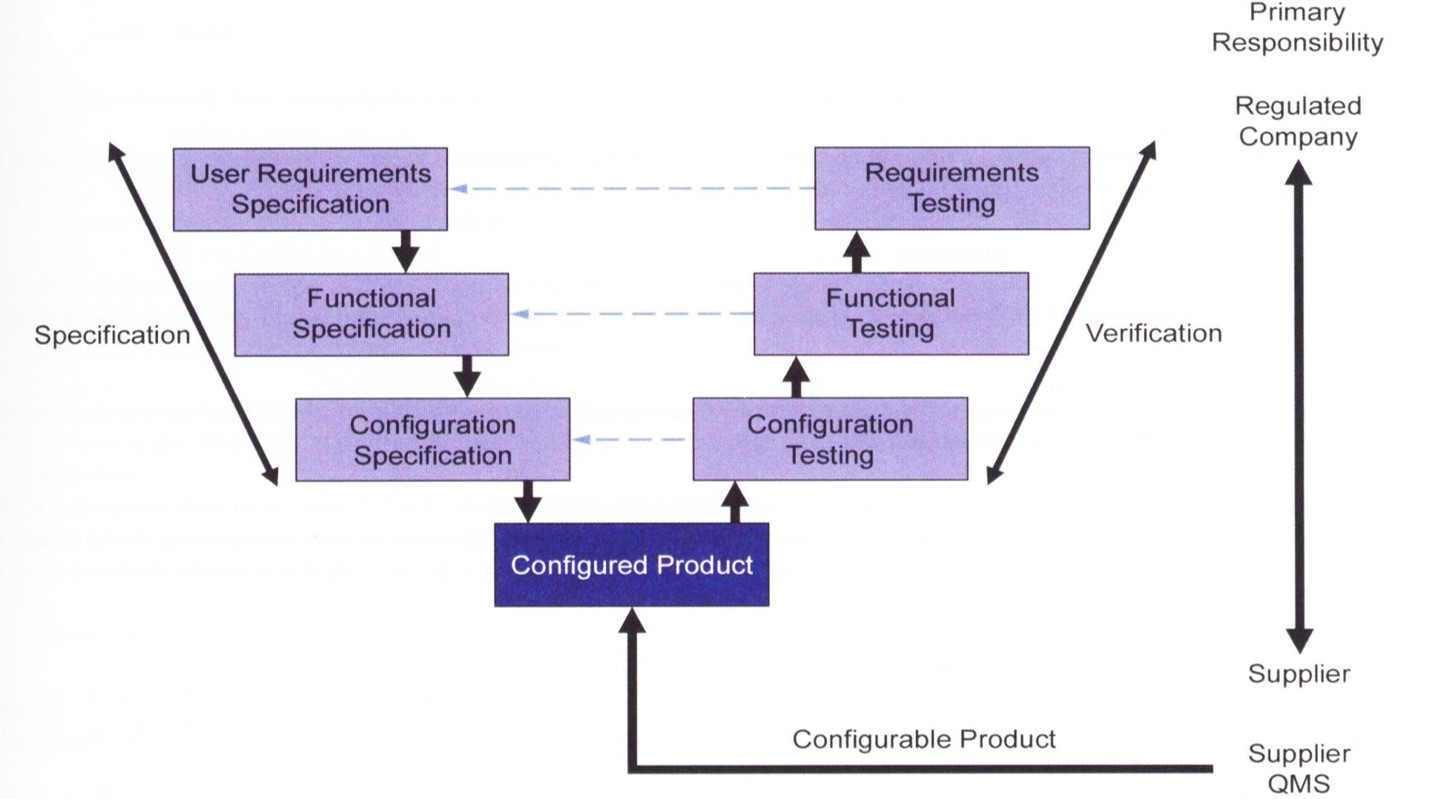
LEVERAGE THE BATCHMASTER FDA VALIDATION TOOLKIT
The BatchMaster FDA Validation Toolkit helps companies deliver consistent, repeatable documentation for auditor review for the initial and subsequent validations (due to system modifications). The Toolkit documentation includes:
- Templates incl. Master Plan, Determination, Risk Assessment, Security, Trace Matrix, Migration Plan, Change Request, Recovery Plan, User Acceptance Test
- Test Scripts incl. Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ)
Leveraging the predefined test scripts, manufacturers can jumpstart their FDA Validation projects by personalizing these test scripts to meet their unique operations, which will then be tested and documented in accordance with the predefined templates. In summary, the toolkit documents are
- Relevant – Required Documentation per Validation Step
- Formatted – TOC of Objective, Sequence/Steps, Sign Offs
- Operational Specific – Based upon Pharmaceutical operations
- Software Specific – Identified BatchMaster ERP processes and transactions
CONTACT US / REQUEST A DEMO
Let’s see if BatchMaster Software has the right software solution for your needs. Tell us about your operations and challenges or request a demo of specific functions.

