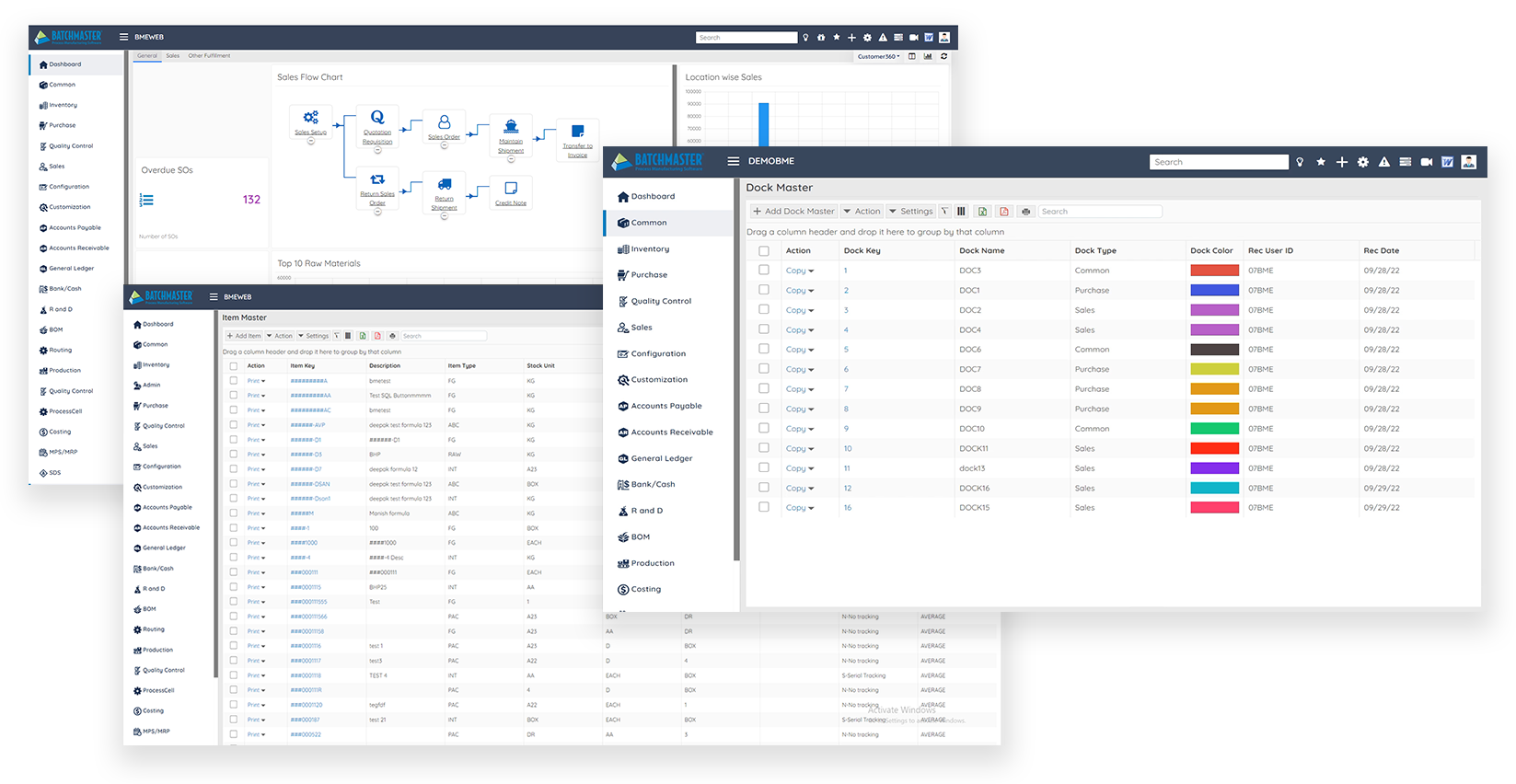
BatchMaster Cloud ERP
The Leader in Process Manufacturing ERP Solutions
BatchMaster Cloud ERP is a complete, web-based ERP solution designed to meet the unique needs of formulaand recipe-based manufacturers. It delivers modern, intuitive, and comprehensive industry-specific solutions for
manufacturers in industries such as:

Food & Beverage

Chemicals

Life Sciences & Pharma
Nutraceuticals

Personal Care & Cosmetics
Paints & Coating
Our Customers








Solution Benefits
- Fully web-based with mobile applications and modern, easy-to-use interface
- Best-in-class, industry-specific functionality with robust quality and compliance features to meet the needs of process manufacturers
- Comprehensive ERP with built-in Finance module and integrations to SAP, QuickBooks, and more
Business Benefits
- Streamline, standardize, and scale up production and business operations
- Improve decision-making with increased visibility, granularity, and real-time data
- Accelerate production cycles and product development
- Ensure compliance and quality that meet stringent regulatory requirements
- Increase inventory turns and minimize expired inventory
- Make agile adjustments to meet dynamic market demands
- Comprehensive product and process traceability for enhanced recall readiness

Core Capabilities

Laboratory & Formula Management
Ensure formula security with approval processes and audit trails, featuring version control, rollback, dynamic reformulation, “what if” analysis, and side-by-side comparisons

Planning & Scheduling (MRP/MPS)
Directly transfer MPS/MRP orders to purchase and production, handle both make-to-stock and make-to-order demands, and auto generate production schedules based on demand.

Batch Production
Dynamically size and prioritize batch jobs. Auto-create production orders from sales orders and adjust formulas before and during WIP
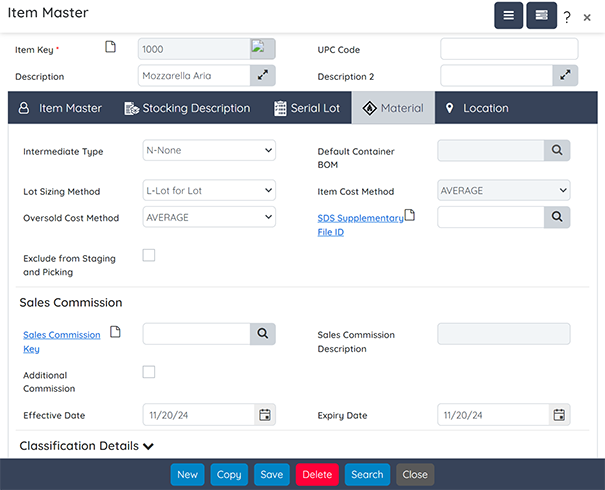
Inventory Management
Manage lot-controlled inventory based on quality status, expiration dates, and potency values. Perform physical counts and inventory revaluation, and track inventory adjustments and movements

Traceability & Compliance
Track products from receipt to shipment and vice-versa, speed up lot recalls and audits, and support requirements such as FDA, 21 CFR Part 11, GFSI and cGMP

Quality Control & Quality Assurance
Establish library of standardized QC tests and sample plans. Define QC tests for formulas, raw materials, intermediates, and finished goods; and analyze results by item, lot, batch job, and vendo

Product Costing
Calculate expected finished good costs, including intermediates and assemblies, based upon cost rollup of ingredients and packaging, and labor, setup, and other production costs

Sales Management
Easily generate sales quotations & orders, handle returns, and transfer quotes to orders, and orders to shipments with one click. Apply volume-based pricing, discounts, and mark-ups

Purchase Management
Create purchase requests and orders, define approvals, and manage multiple pricelists for purchases, returns, and agreements, ensuring cGMP materials are sourced from specific vendors

Finance
Streamline financial operations by maintaining ledger entries, tax calculations, and multi-currency transactions while integrating with purchasing and sales to enhance cash flow visibility